Abstract
Background: Multi-centered longitudinal observational and interventional trials over five decades have shifted SCD from a disease of childhood to a disease of young and middle-aged adults, with an average life expectancy extending into the fifth decade. However, the aging SCD patient, who may have faced early mortality decades ago, now faces novel, complex, and uncharacterized disease manifestations in an era of fragmented care and little coordination. Single-center studies, limited to local institutional data sets, lack sufficient breadth to inform evidence-based clinical guidelines, and the natural history of the patient with SCD in the current era is not known. Web-based data collection and the electronic health record (EHR) have the potential to transform SCD care in the US. Using these tools, providers at multiple academic health centers together developed a multi-centered prospective database to characterize the expanding transition population and adult cohort in the modern era. Our objective is to demonstrate the feasibility of this prospective collaborative data network, the (GRNDaD) registry.
Methods: GRNDaD is recruiting adult and pediatric patients at four sites (Baltimore, Cleveland, Milwaukee, and Oakland, Ca.). Through an iterative process using existing registries at the participating sites and broadly validated measures (PhenX), a set of variables were agreed upon by the site investigators and created in REDCap. The full registry has 117 unique variables from the medical record, 72 of which are mandatory during annual updates. Survey data, including ASCQ-Me and the brief pain inventory, are available for direct patient entry. A detailed codebook with variable definitions has been developed. The registry includes an output template that provides patient specific routine health maintenance information for review during clinical care. Here, we report on 166 participants with a minimum dataset entered over 6 months, estimated to be 15% of the cumulative population at our sites. All lab studies entered are those measured at a routine follow-up visit. Ttests and chi -square were used to compare differences in lab values and complications between those with sickle cell anemia (SCA) and variant sickle hemoglobinopathies.
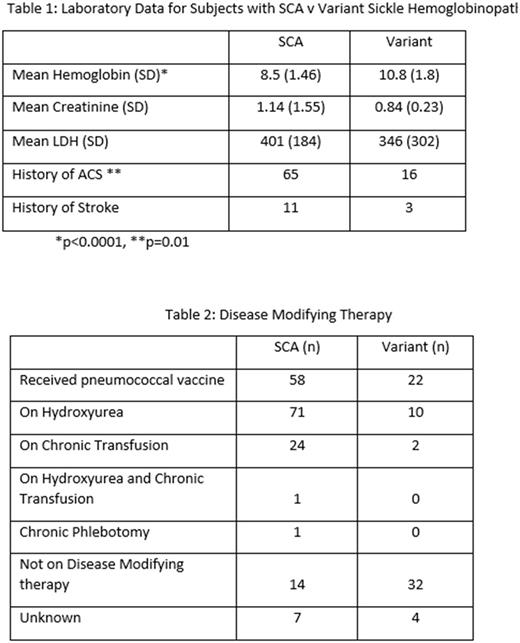
Results: 471 patients consented to the registry, with <5% refusal (n=18). Those with full data in the registry includes 118 patients with phenotypic SCA, with an average age of 35.4 years. There are 48 patients with sickle variants: 27 with SC, 18 with Sβ+ Thalassemia, and 3 others, with an average age of 38.5 years. 48% of participants had documentation of pneumococcal vaccination. Notably, 82% of patients with SCA are on disease modifying therapy. Likely as a consequence, LDH values do not differ significantly between SCA and variant SCD in GRNDaD, which is not seen in other registry reports. Lab values and disease modifying therapy can be seen in Tables 1 and 2.
Conclusions: These data represent care provided at the four comprehensive sickle programs involved in this project. Two additional sites are joining GRNDaD in late 2017, and the goal is to continue to expand. Using a web-available user-friendly portal, prospective registry data can be collected in a timely fashion at multiple centers. Currently data are extracted manually, but code is being developed to extract data directly from the EHR to improve efficiency. This will provide a unique snapshot of the current state of care of large groups of individuals with SCD. Registry participation is well received by potential subjects. One limitation identified early is that vaccinations may be provided outside of the comprehensive center (in primary care clinics) and so alternative strategies may be needed to reliably collect this data. Patient-specific data output from GRNDaD, for use clinically, is being optimized. Over time, as we collect data on important outcomes, we anticipate that GRNDaD will allow the recognition of novel phenotypic subpopulations and risk profiles, and will optimize clinical management, therapeutic product development, and quality assurance for our patients.
Lanzkron: Pfizer: Research Funding; Global Blood Therapeutics: Research Funding; Bayer: Research Funding; HRSA: Research Funding; Prolong: Research Funding; PCORI: Research Funding. Field: NKT Therapeutics: Honoraria, Research Funding; Astellas: Research Funding; Prolong Pharmaceuticals: Research Funding; Incyte: Research Funding. Hoppe: Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Little: Hemex Health: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal